The study of spermatogenesis

Chapter 1: Spermatogenesis
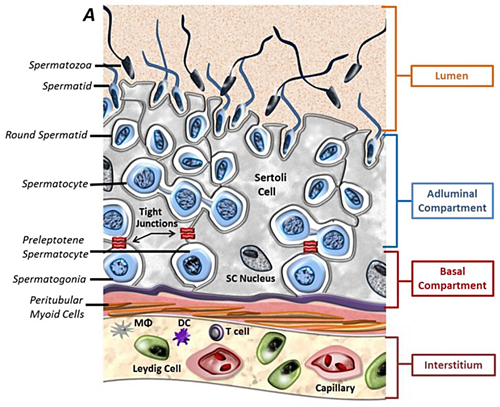
Cellular components of the testis. Testis interstitium consists of Leydig cells, macrophages (MΦ), tolerogenic dendritic cells (DC), T cells and blood vessels. The seminiferous tubules are composed of Sertoli cells and maturing germ cells surrounded by peritubular myoid cells (Kaur G et al., Semin Cell Dev Biol, 2014).
Spermatogenesis is fundamental to the establishment and maintenance of male fertility, while abnormal spermatogenesis will lead to male infertility/subfertility or various testicular tumors. It is generally considered that mammalian spermatogenesis is a complex sequential process of germ cell differentiation from primordial germ cells or spermatogonial stem cells to functional haploid sperm [1]. In addition to germ cell development, spermatogenesis requires significant contributions of somatic cell populations, including testicular Sertoli cells, Leydig cells, peritubular myoid cells, blood vessels, and macrophages. The importance of Sertoli cells, Leydig cells, and macrophages in spermatogenesis has been fully revealed via cell ablation strategy and conditional knockout mouse models [2, 3]. However, the regulatory roles and mechanism of peritubular myoid cells in testis development and spermatogenesis remain largely unknown.
Chapter 2: Peritubular myoid cells
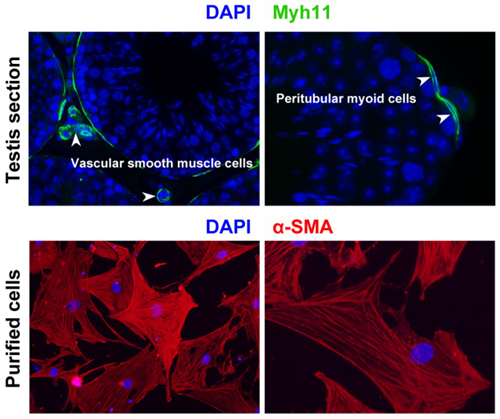
Peritubular myoid cell localization. In mouse adult testis, smooth muscle cell marker Myh11 expression is restricted to peritubular myoid cells and vascular smooth muscle cells. Peritubular myoid cells can be isolated using Percoll discontinuous gradients and further identified by immunostaining of another smooth muscle cell marker α-SMA (Wang YQ et al., Reproduction, 2018).
Peritubular myoid cells are the main cellular components of the wall of seminiferous tubules. They possess smooth muscle-like characteristics and are thought to be important for the intratesticular transport of immotile sperm. Loss of contractility in human peritubular myoid cells may contribute to sub- or infertility, because peritubular myoid cell markers, such as myosin heavy chain (Myh11) and alpha smooth muscle actin (α-SMA) are often lost or diminished in peritubular myoid cells of men with impaired spermatogenesis. Recent studies have shown that several peritubular myoid cell-expressed genes, such as colony stimulating factor 1 (Csf1), androgen receptor (AR), leucine-rich repeat-containing G protein-coupled receptor 4 (Lgr4), and glial cell line-derived neurotrophic factor (Gdnf), are essential for mouse spermatogenesis by knockout mouse models [4-7]. Furthermore, contraction and relaxation of peritubular myoid cells are regulated by sympathetic innervation and paracrine and endocrine substances, including endothelin (ET-1), angiotensin II (AngII), platelet-derived growth factor (Pdgf-BB), prostaglandin F2α (Pgf2α), neurotransmitters, and hormones [8]. However, the overall role of peritubular myoid cells and individual function of peritubular myoid cell-expressed genes remain poorly understood.
Bottleneck
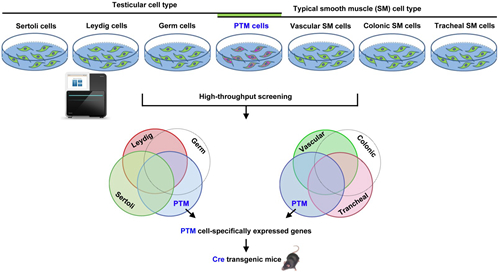
Exploring a PTM cell-specific marker. Peritubular myoid (PTM) cells are smooth muscle-like cells. Differentially-expressed gene screening among different testicular cell type (e.g. Sertoli cells, Leydig cells, germ cells and PTM cells) and typical smooth muscle (SM) cell type (vascular SM cells, colonic SM cells, tracheal SM cells and PTM cells) could be helpful to explore a PTM cell-specific marker and generate its Cre transgenic mice.
Now, testicular peritubular myoid cell-specific gene knockout is impossible because no markers exist to identify peritubular myoid cells yet. The Myh11-Cre mouse strain was used to remove AR and Gdnf within peritubular myoid cells in previous studies [5, 7]. However, Myh11-Cre is expressed in all kinds of smooth muscle cells, including, but not limited to peritubular myoid cells [9]. It means that researchers have to confirm the absence of their interested genes in testicular vascular smooth muscle cells and other body smooth muscle cells before applying Myh11-Cre-mediated conditional knockout strategy. Accordingly, there is a pressing need to discover a peritubular myoid cell-specific marker and generate peritubular myoid cell-specific Cre transgene.
Reference
[1] Chen SR & Liu YX 2015 Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction 149 R159-167.
[2] Rebourcet D, O'Shaughnessy PJ, Pitetti JL, Monteiro A, O'Hara L, Milne L, Tsai YT, Cruickshanks L, Riethmacher D, Guillou F, et al. 2014 Sertoli cells control peritubular myoid cell fate and support adult Leydig cell development in the prepubertal testis. Development 141 2139-2149.
[3] DeFalco T, Potter SJ, Williams AV, Waller B, Kan MJ & Capel B 2015 Macrophages contribute to the spermatogonial niche in the adult testis. Cell Rep 12 1107-1119.
[4] Oatley JM, Oatley MJ, Avarbock MR, Tobias JW & Brinster RL 2009 Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 136 1191-1199.
[5] Welsh M, Saunders PT, Atanassova N, Sharpe RM & Smith LB 2009 Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J 23 4218-4230.
[6] Qian Y, Liu S, Guan Y, Pan H, Guan X, Qiu Z, Li L, Gao N, Zhao Y, Li X, et al. 2013 Lgr4-mediated Wnt/beta-catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development 140 1751-1761.
[7] Chen LY, Willis WD & Eddy EM 2016 Targeting the Gdnf Gene in peritubular myoid cells disrupts undifferentiated spermatogonial cell development. Proc Natl Acad Sci U S A 113 1829-1834.
[8] Mayerhofer A 2013 Human testicular peritubular cells: more than meets the eye. Reproduction 145 R107-116.
[9] Chen SR & Liu YX 2016 Myh11-Cre is not limited to peritubular myoid cells and interaction between Sertoli and peritubular myoid cells needs investigation. Proc Natl Acad Sci U S A 113 E2352.
