Studies in the regulation of early pregnancy

Studies the regulation of early pregnancy
Early pregnancy establishment, especially successful of embryo implantation, is the cornerstone of reproduction, and determines the pregnancy outcome. It has been reported that 75% of pregnancy losses are rooted in peri-implantation period. Our lab studies the regulation of early pregnancy including: how the embryo develops into normal blastocyst and transports into uterus timely; how uterus gets the receptivity; and the precise communication of embryo and uterus. We wanted to reveal the key regulator and mechanism in early pregnancy establishment from mouse model, that is close to our own, human pregnancy.
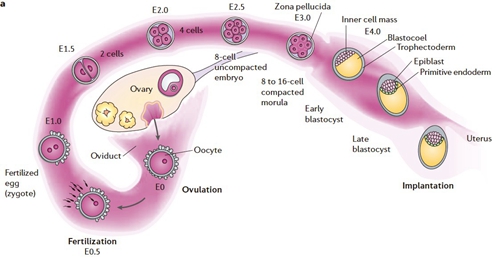
Figure 1 Overview of the process of human early pregnancy (sited from Nat Rev Genet, 2006)
Focus 1 Regulation of embryo and uterus communication
After blastocyst enters into uterus, the crosstalk between an embryo and the uterus determines when and where the embryo implants into the uterus. A precisely regulated intrauterine environment is responsible for pregnancy establishment, and disruption of the uterine fluid homeostasis during this process can cause abnormal embryo implantation and pregnancy loss. We wanted to identify the key molecules in uterine environment that mediate blastocyst and uterus communication.
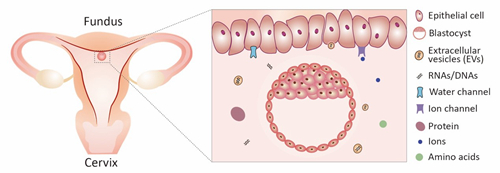
Figure 2 uterine fluid environment mediates the crosstalk between an embryo and uterus.
Focus 2 Maternal environmental exposure epigenetically influence embryo development and fetus health
Certain maternal lifestyle factors, such as environmental exposure and nutritional condition, can directly affect embryonic development and result in offspring metabolic disorders and disease predispositions. However, how maternally acquired environmental traits influence a developing embryo in the uterus remains unresolved. Peri-implantation is a critical time window during which the developing embryo can be regulated or affected by active molecules in the uterus and we propose that maternal environmental exposure during this period may be transferred to an embryo, which might result in long-term effects on offspring well-being.
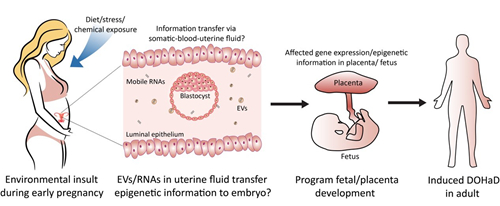
Figure 3 Maternal environmental exposure might epigenetically influence embryo development and fetus health
Focus 4 New technology of reproduction study
Reproduction is a traditional field, but with technological development, new studying methods which are applied into recent studies, rekindled great interests in this old yet fascinating field. For example, deep imaging provides more information about reproductive system and bring more possibility to open the black box of fertilization and pregnancy.
Selected publication:
1. Qian J, Zhang Y, Qu Y, Zhang L, Shi J, Zhang X, Liu S, Bo H, Sung J, Zhou T, Chen Q, Sean M, Duan E*, Zhang Y*. Caffeine consumption during early pregnancy impairs oviductal embryo transport, embryonic development and uterine receptivity in mice. Biol Reprod. 2018. Jul 5. doi: 10.1093/biolre/ioy155.
2. Zhang Y#, Zhang X#, Shi J#, Tuorto F#, Li X#, Liu Y, Liebers R, Zhang L, Qu Y, Qian J, Pahima M, Liu Y, Yan M, Cao Z, Lei X, Cao Y, Peng H, Liu S, Wang Y, Zheng H, Woolsey R, Quilici D, Zhai Q, Li L, Zhou T, Yan W, Lyko F, Zhang Y*, Zhou Q*, Duan E*, Chen Q*. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat Cell Biol. 2018. 20, 535–540.( Highlighted in Nat Rev Endo.)
3. Zhang Y#, Wang Q#, Wang H, Duan E. Uterine Fluid in Pregnancy: A Biological and Clinical Outlook. Trends Mol Med. 2017. May 25. pii: S1471-4914(17)30080-1. (Highlighted in Cell Press & highlighted in F1000)
4. Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, Feng G, Peng H, Zhang X, Zhang Y, Qian J, Duan E*, Zhai Q*, Zhou Q*. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016 Jan 22;351(6271):397-400
5. Zhang Y#, Chen Q#, Zhang H#, Wang Q#, Li R, Jin Y, Wang H, Ma T, Qiao J, Duan E. Aquaporin-dependent excessive intrauterine fluid accumulation is a major contributor in hyper-estrogen induced aberrant embryo implantation. Cell Res. 2015. Jan;25(1):139-42.
6. Shi J, Chen Q, Li X, Zheng X, Zhang Y, Qiao J, Tang F, Tao Y, Zhou Q, Duan E. (2015) Dynamic transcriptional symmetry-breaking in pre-implantation mammalian embryo development revealed by single-cell RNA-seq. Development. 2015 142, 3468-3477.
7. Zhang Y#, Zhang Y#, Shi J#, Zhang H, Cao Z, Gao X, Ren W, Ning Y, Ning L, Cao Y, Chen Y, Ji W, Chen ZJ, Chen Q, Duan E. Identification and characterization of an ancient class of small RNAs enriched in serum associating with active infection. J Mol Cell Biol, 2014. 6 (2): 172-174. (Cover Story & Editor’s Recommendation)
8. Chen Q#, Zhang Y#, Elad D, Jaffa AJ, Cao Y, Ye X, Duan E. Navigating the site for embryo implantation: biomechanical and molecular regulation of intrauterine embryo distribution. Mol Aspects Med, 2013. Oct;34(5):1024-42.
9. Chen Q#, Zhang Y#, Peng H, Lei L, Kuang H, Zhang L, Ning L, Cao Y, Duan E. Transient {beta}2-adrenoceptor activation confers pregnancy loss by disrupting embryo spacing at implantation. J Biol Chem. 2011.286: 4349-4356.
