Identification of key genes and pathways that control X status and XCI in early human embryos.
Date:2020-05-07 [close]
In mammals, X chromosome inactivation is an important process for balancing gene expression level between the female cells with two X chromosomes and the male cells with a single X chromosome [1]. Inactivation of the X chromosome can be confirmed by identifying a long non-coding RNA Xist. Early studies on mouse embryos found that the X chromosome inactivatioin(XCI) process presents a way of imprinting inactivation [2]. In the mouse fertilized egg, the two X chromosomes are both activated; in the 4-8 cell stage, the paternal X chromosome is imprinted inactivated, while the maternal X chromosome remains activated, and the state is maintained until in the trophectoderm. However, in the inner cell mass of the blastocyst stage, the paternal X chromosome is reactivated and the two activated X chromosomes are randomly inactivated with subsequent differentiation. In vitro, mouse embryonic stem cells derived from the inner cell mass also possess two activated X chromosomes, and have no XIST expression, consistent with the state in vivo [3]. Unlike mice, XCI occurs in late blastocyst stages or after implantation in human early embryos. In the inner cell mass of the E7 phase, the two X chromosomes are activated, but at the same time accompanied by high expression of XIST [4, 5]. However, the human embryonic stem cells obtained by isolating from the inner cell mass have different X chromosome states with in vivo. XCI has occurred in most cells, and the inactive X chromosome is enveloped by highly expressed XIST. Only a small fraction of cells are similar to mouse embryonic stem cells and have two activated X chromosomes. Moreover, with the continuation of in vitro culture, a part of the inactivated X chromosome will gradually lose the ability to express XIST [6, 7]. Recently, Dr. Jaenisch R. group established the naive hESC which has more primitive pluripotency than the existing human embryonic stem cell lines, and showed that the cells had two activated X chromosomes, whereas XIST expression was not the same as human early embryos [8]. Therefore, our lab is commited to optimizing naive hESC culture and differentiation protocols, and performing genetic screen to identify key genes and pathways that control X status and XCI in early human embryos.
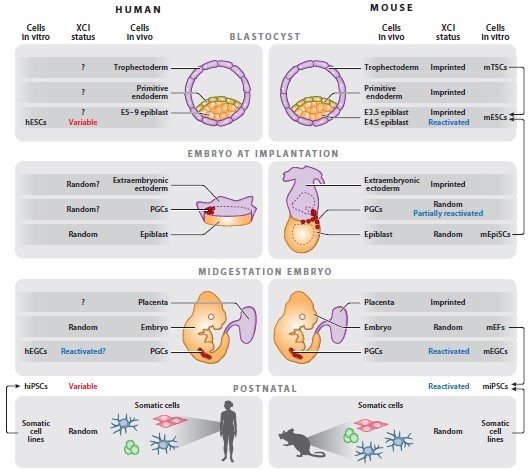
Figure cited from Derek L, Montserrat CA, Jeannie TL. (2013). Annu. Rev. Genomics Hum. Genet, 14:85-110.
References:
1. Disteche, C.M. (2016). Dosage compensation of the sex chromosomes and autosomes. Semin. Cell Dev. Biol. 56:9-18.
2. Mak W, Nesterova TB, de Napoles M, Appanah R, Yamanaka S, Otte AP, Brockdorff N. (2004). Reactivation of the paternal X chromosome in early mouse embryos. Science. 303:666-669.
3. Derek L, Montserrat CA, Jeannie TL. (2013). X chromosome Inactivation and epigenetic responses to cellular reprogramming. Annu. Rev. Genomics Hum. Genet.14:85-110.
4. Okamoto I, Patrat C, Thépot D, Peynot N, Fauque P, Daniel N, Diabangouaya P, Wolf JP, Renard JP, Duranthon V, and Heard E. (2011). Eutherian mammals use diverse strategies to initiate X-chromosome inactivation during development. Nature. 474:239-240.
5. Petropoulos S,Edsg?rd D, Reinius B, Deng Q, Panula SP, Codeluppi S, Plaza Reyes A, Linnarsson S, Sandberg R, Lanner F. (2016). Single-cell RNA-Seq reveals lineage and X chromosome dynamics in human preimplantation embryos. Cell. 165:1012-1026.
6. Nichols, J., and Smith, A. (2009). Naive and primed pluripotent states. Cell Stem Cell. 4:487-492.
7. Lessing D, Anguera MC, Lee JT. (2013). X chromosome Inactivation and epigenetic responses to cellular reprogramming. Annu Rev Genomics Hum Genet. 14:85-110.
8. Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, Lungjangwa T, Imsoonthornruksa S, Stelzer Y, Rangarajan S, D'Alessio A, Zhang J, Gao Q, Dawlaty MM, Young RA, Gray NS, Jaenisch R. (2014). Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 15:524-526.